About EUnetHTA 21
About EUnetHTA 21
EUnetHTA was established to create an effective and sustainable network for HTA across Europe – we work together to help develop reliable, timely, transparent, and transferable information to contribute to HTA in European countries.
EUnetHTA supports collaboration between European HTA organisations that brings value at the European, national, and regional level through:
- The facilitation of efficient HTA resource use.
- The creation of a sustainable system of HTA knowledge sharing.
- The promotion of good practice in HTA methods and processes.
On 17 September 2021, the European Health and Digital Executive Agency (HaDEA) signed the Service Contract for the Provision of Joint Health Technology Assessment (HTA) Work Supporting the Continuation of EU Cooperation on HTA. The contract will run for 24 months, and until 16 September 2023.
EUnetHTA 21 work will build on the achievements and lessons learned from the EUnetHTA Joint Actions and focus on supporting a future EU HTA system under the HTA Regulation. For all EUnetHTA 21 deliverables the future EU HTA Regulation will serve as a basis.
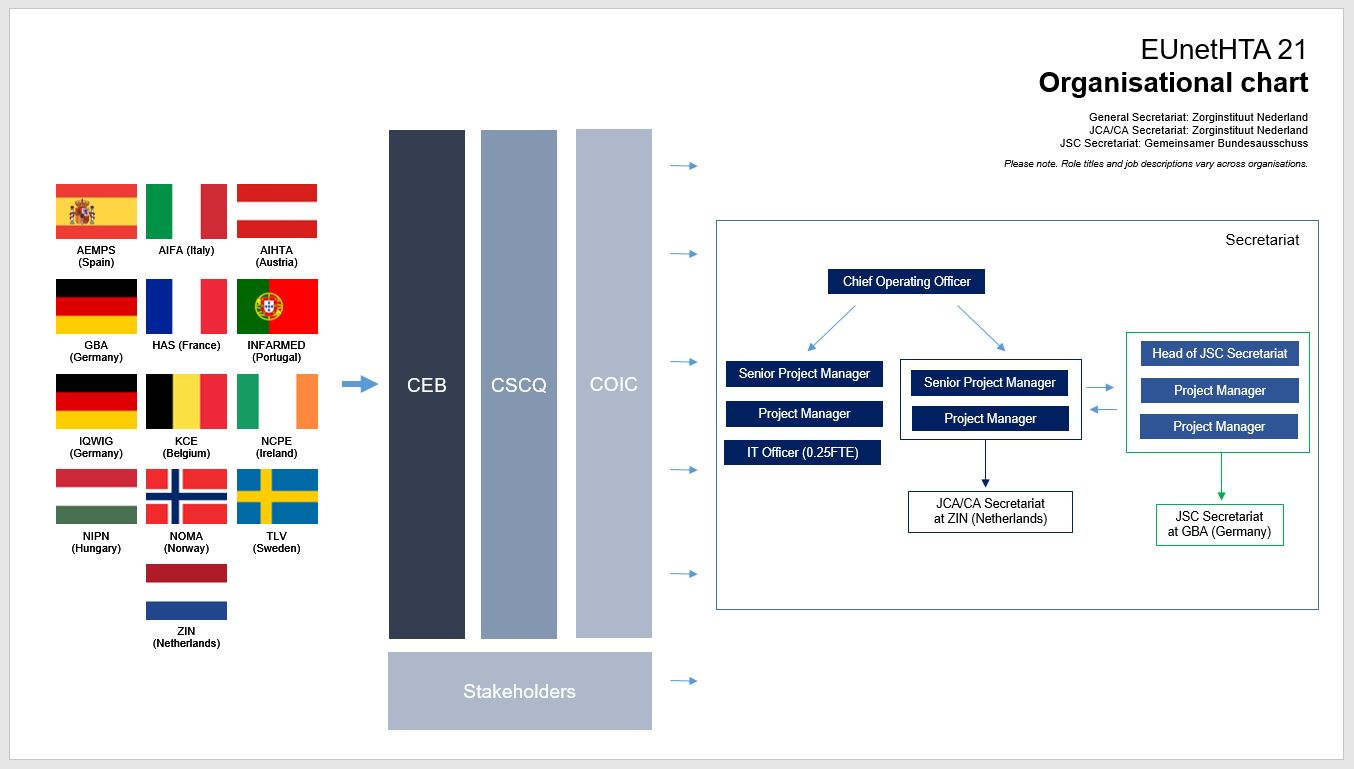
The EUnetHTA 21 joint consortium is led by ZIN (The Netherlands) and includes the following HTA agencies: AEMPS (Spain), AIFA (Italy), AIHTA (Austria), GBA (Germany), HAS (France), INFARMED (Portugal), IQWIG (Germany), KCE (Belgium), NCPE (Ireland), NIPN (Hungary), NOMA (Norway) and TLV (Sweden).