OCTA20 Final Assessment Report is now available.
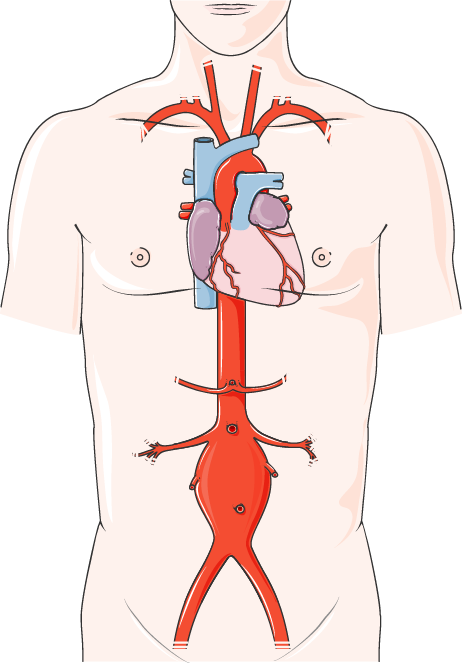
We are pleased to announce that the final assessment report for OTCA20 ‘Prophylactic or therapeutic use of endoanchoring systems in endovascular aortic aneurysm repair (EVAR/TEVAR)’, together with the fact check comments from manufacturers and comments from external experts including the replies of the author, are now available for access. The health technology assessed is a […]
OCTA20 Final Assessment Report is now available.

We are pleased to announce that the final assessment report for OTCA20 ‘Prophylactic or therapeutic use of endoanchoring systems in endovascular aortic aneurysm repair (EVAR/TEVAR)’, together with the fact check comments from manufacturers and comments from external experts including the replies of the author, are now available for access. The health technology assessed is a […]
OTCA18 Final Assessment Report is now available.

The final assessment report and related documents concerning OTCA18, ‘regional hyperthermia for high-risk soft tissue sarcoma treatment’, are now available. The assessed technology is regional hyperthermia added to conventional therapies to treat high-risk soft tissue sarcoma. The objective of this rapid relative effectiveness assessment was to collaboratively address the research question: Is the regional application […]
Open Call for Patient Input for a new Joint Assessment on a medicinal product for neuromyelitis optica spectrum (NMOSD) disorders.

Patient group input requested for a new Joint Assessment on a medicinal product for neuromyelitis optica spectrum disorders. EUnetHTA deems patient involvement very important in the production of Joint Assessment reports. We recognise that patients and those who support them have unique knowledge about what it is like to live with a specific disease or […]
PTJA07 Final Assessment Report is now available

We are pleased to announce that the Final Assessment Report for PTJA07 is now available, with supporting documentation, for access. Please follow this link for full access.
EUnetHTA Magazine – Autumn 2019 – Now Available
We are pleased to publish the Autumn 2019 issue of EUnetHTA Magazine. Intended for improved accessibility on whichever device you use, this issue includes various contributions from the EUnetHTA member network, while updating information on EUnetHTA products and the state of HTA in Europe. As always, we welcome your feedback so we can continue to […]
Minutes from the EMA-EUnetHTA July 2019 Meeting now available
The minutes from the EMA-EUnetHTA Bilateral Meeting, hosted by the EUnetHTA at the Zorginstituut Nederland (ZIN) in Diemen, Netherlands, on 4th July, 2019, are now available for access. Topics discussed included recent experiences with exchanges on medicinal products, practices and experiences related to patient involvement, and compliance with post marketing activities. The minutes can be […]
Open Call for Patient Input for a new Joint Assessment on a medicinal product for acute myeloid leukaemia.

Patient group input requested for a new Joint Assessment on a medicinal product for acute myeloid leukaemia. EUnetHTA deems patient involvement very important in the production of Joint Assessment reports. We recognise that patients and those who support them have unique knowledge about what it is like to live with a specific disease or […]
REQueST Tool and its vision paper are now available for access.
The Registry Evaluation and Quality Standards Tool (REQueST) aims to support HTA organisations and other actors in guiding and evaluating registries for effective usage in HTA. Following public consultation, a finalised version of the tool, together with its vision paper, a document that explores the options for the long-term delivery, use and sustainability of REQueST […]
PTJA12 – EUnetHTA is pleased to announce the start of the next Pharmaceutical Joint Assessment.

EUnetHTA is pleased to announce the start of the next Pharmaceutical Joint Assessment. PTJA12 addresses ‘Glasdegib indicated for the treatment of newly diagnosed de novo or secondary acute myeloid leukaemia (AML)’, submitted by Pfizer. We are delighted that HVB and NCPE are authoring this assessment. We will be issuing an Open Call for Patient Input […]

