The Joint Assessment, OTJA08, on “Continuous glucose monitoring (CGM real-time) and flash glucose monitoring (FGM) as personal, standalone systems in patients with diabetes mellitus treated with insulin” is now available.

We are pleased to announce that the joint rapid assessment on “Continuous glucose monitoring (CGM real-time) and flash glucose monitoring (FGM) as personal, standalone systems in patients with diabetes mellitus treated with insulin” is available. The health technologies assessed include rtCGM systems and FGM systems as personal, standalone medical devices for patients with diabetes mellitus […]
OTJA10 “Stool DNA testing for early detection of colorectal cancer” Final Project Plan Now Available

[vc_row][vc_column][vc_column_text]This is the final project plan of the assessment on Stool DNA testing for early detection of colorectal cancer. Download Final Project Plan HERE Download summary of comments received from external review, including the authors’ answers HERE [pdf-embedder url=”https://www.eunethta.eu/wp-content/uploads/2018/07/OTJA10_Project-Plan__final.pdf” title=”OTJA10_Project Plan__final”][/vc_column_text][/vc_column][/vc_row]
OTCA14 “Robotic surgery in thoracic and visceral indications” Final Project Plan and Combined Comments Now Available

This is the final project plan of the assessment on robotic surgery in thoracic and visceral indications. The combined comments from external experts and the manufacturer fact check with authors’ replies are also available. Download the final project plan HERE Download the combined comments and fact check HERE [pdf-embedder url=”https://www.eunethta.eu/wp-content/uploads/2018/07/Project-Plan_OTCA14.pdf” title=”Project Plan_OTCA14″] [pdf-embedder url=”https://www.eunethta.eu/wp-content/uploads/2019/05/Combined-comments-from-external-experts-and-manufacturer-fact-check-with-authors-replies-OTCA14.pdf” title=”Project […]
Patient group input requested for a new Joint Assessment on a medicinal product for Diabetes Type 1 – Closing August 3

EUnetHTA Joint Production is pleased to announce the launch of the REA for Sotagliflozin for Type 1 diabetes mellitus (PTJA04) For this specific assessment on a medicinal product for Diabetes Type 1, an ‘open call for patient group submission‘ is used. We are seeking for European and national patient organisations to provide an organisational perspective […]
Materials now available from 1st EUnetHTA Workshop on HTA and MDR/IVDR (from 29th May 2018, Vienna) and 2nd Workshop Announced (28 May, 2019, Vienna)

We are happy to announce that the documentation of the 1st EUnetHTA Workshop on HTA and MDR/IVDR (from 29th May 2018, Vienna) is now available. Please note that the 2nd EUnetHTA Workshop will take place on TUE 28th May 2019 in Vienna. For further information please contact EUnetHTA_MedDevice_Event@hta.lbg.ac.at [pdf-embedder url=”https://www.eunethta.eu/wp-content/uploads/2018/07/Workshop1_Documentation_05.07.2018.pdf”]
September 3, 2018 – Real-World Evidence in Medicine Development – An online introductory course – call for registration
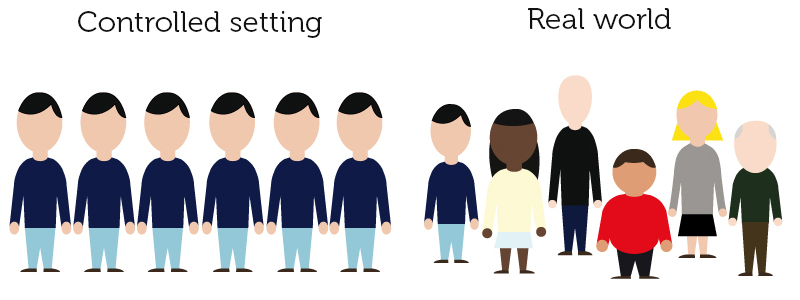
Real-World Evidence in Medicine Development An online introductory course Under the supervision of leading academics involved in the IMI GetReal project, this interactive online course will give an understanding of current techniques, opportunities and challenges for the use of real-world evidence in medicine development. This is an ideal course for anyone who wishes to become […]
Quality Survey – 2018 EUnetHTA Forum, Cologne
Attended or participated in the live web stream of the the 2018 EUnetHTA Forum in Cologne? We’d love to hear from you. Closing July 20.
Public Consultation – Early Dialogues for Medical Device Developers

Following on the successful launch of the JA3 procedures for European Network for Health Technology Assessment (EUnetHTA) Early Dialogues (ED) for Pharmaceutical products, EUnetHTA is pleased to announce the future launch of EDs for Medical Devices. The procedure is a single gateway for early dialogues with European HTA bodies on their evidence-generation plans for medical […]
National Implementation Report Now Available
[vc_row][vc_column][vc_column_text]The first National Implementation report has now been published. The report follows up the first five EUnetHTA assessments to be published. For these assessments, 46 examples of use have been reported, including both use to support assessment procedures and use as part of dissemination to support awareness of HTA for decision making purposes. As well […]
Registration now open – The way forward for HTA cooperation – the views of stakeholders

Brussels, July 9, 2018 – Registration is now open Programme Registration Venue Programme Please see the draft agenda of the event•••. Registration Registration to the event is available here. To access to this registration page, you should be registered in the European Commission Access System – EULogin. Please note that all registrations need to be confirmed by the […]
