EUnetHTA 21

On 17 September 2021, the European Health and Digital Executive Agency (HaDEA) signed the Service Contract for the Provision of Joint Health Technology Assessment (HTA) Work Supporting the Continuation of EU Cooperation on HTA. The contract will run for 24 months, and until 16 September 2023. EUnetHTA 21 work will build on the achievements and lessons […]
RCROT02 – Rapid Collaborative Review on the diagnostic accuracy of molecular methods that detect the presence of SARS-CoV-2 virus in people with suspected COVID-19 – report now available

EUnetHTA is pleased to announce that the “Rapid Collaborative Review on the diagnostic accuracy of molecular methods that detect the presence of SARS-CoV-2 virus in people with suspected COVID-19” is now available. This is the second rapid review assessing health technologies for SARS-CoV-2. The objective of this assessment was to identify, assess and summarise evidence on […]
EUnetHTA Covid-19 Response

To help in the development of a coordinated response to the Covid-19 pandemic, it is essential that researched, timely and reliable information can be made available to all stakeholders, whether healthcare professionals or members of the public. With this in mind, EUnetHTA has taken the decision to prioritise Covid-19-related initiatives above other work for the […]
EUnetHTA Magazine – Autumn 2019 – Now Available
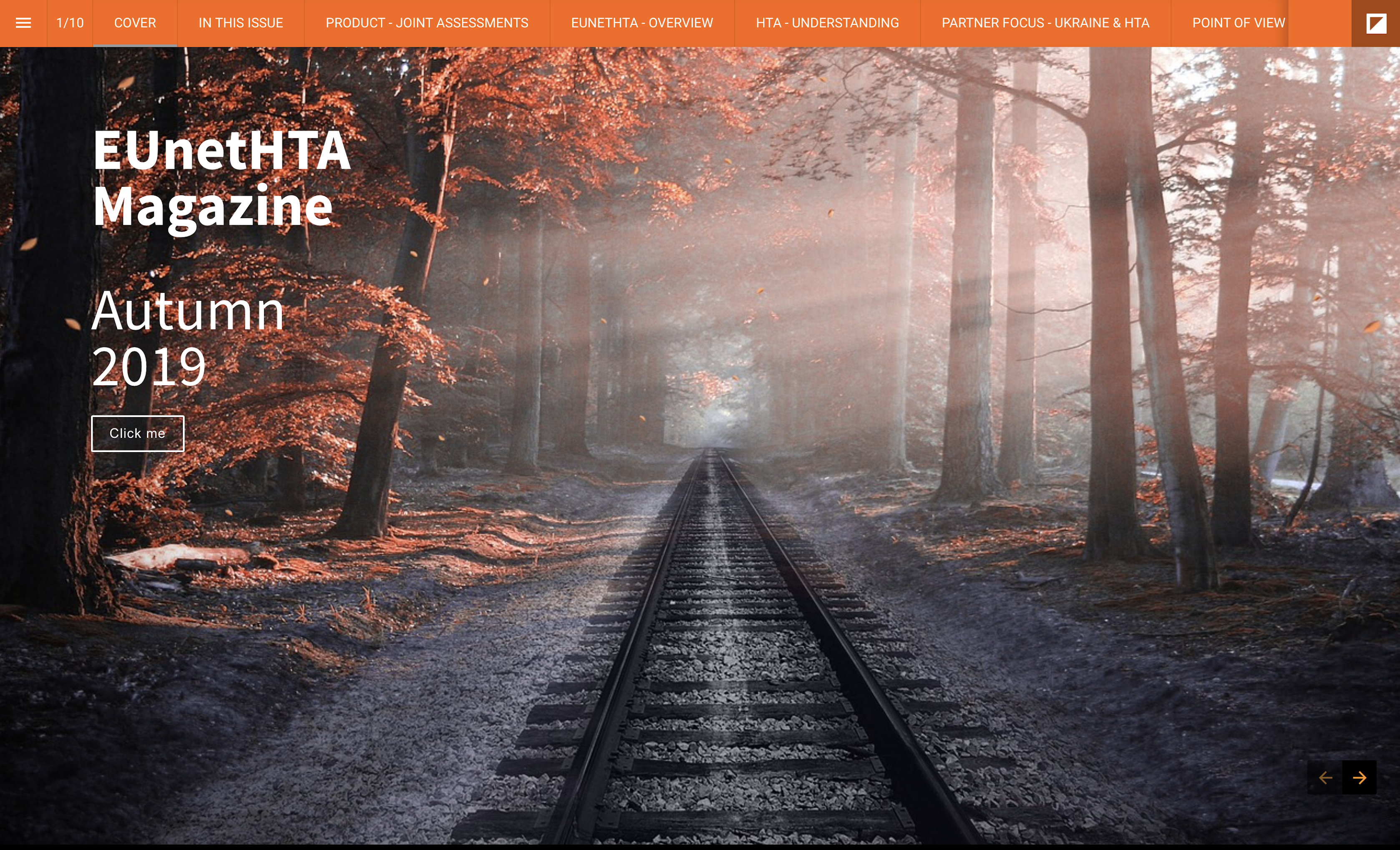
We are pleased to publish the Autumn 2019 issue of EUnetHTA Magazine. Intended for improved accessibility on whichever device you use, this issue includes various contributions from the EUnetHTA member network, while updating information on EUnetHTA products and the state of HTA in Europe. As always, we welcome your feedback so we can continue to […]
PTJA07 Pharmaceutical Joint Assessment Now Available – ustekinumab for the treatment of adult patients with moderately to severely active ulcerative colitis (uc) who have had an inadequate response with, lost response to or were intolerant to either conventional therapy or a biologic or have medical contraindications to such therapies.

This is the pharmaceutical Joint Assessment PTJA07 – on ustekinumab for the treatment of ulcerative colitis (UC). In September 2019, EMA approved the extension of the indication for ustekinumab to include treatment of adult patients with moderately to severely active UC who have had an inadequate response with, lost response to or were intolerant to […]
EUnetHTA Magazine – Winter 2017-2018

Interview with Menno Aarnout/AIM, Partner Profiles: Portugal, Impact: Croatia and Italy, A Patient’s Perspective: Dr. Cees Smit, EMA-EUnetHTA Finalise Joint Work Plan, 2017-2020 winter_2017_magazine_final

