OTCA15 – Final Assessment and input from external experts and manufacturers are now available
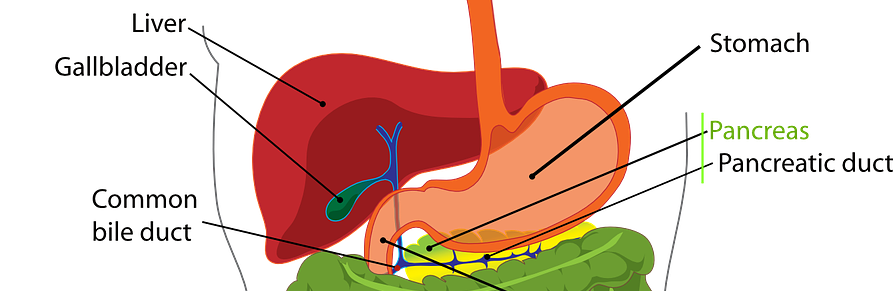
We are pleased to announce that the final assessment for OTCA15 “Irreversible electroporation in liver and pancreatic cancer”, together with the input from external experts and manufacturers, are now available for access. Irreversible electroporation (IRE) is a nonthermal ablative method based on the application of short high-voltage and low-frequency electric fields to destroy tissue. The purpose of […]
EUnetHTA Magazine – Summer 2019 – Now available

We are pleased to roll out the next issue of EUnetHTA Magazine. As we are now experimenting with a web-based format, we hope to provide a more interactive experience for readers, whether that’s on desktop, tablet, or mobile device. In this issue we have healthcare thoughts from Finland, updates from MD and an interview with […]
”Patient Input in Relative Effectiveness Assessments” is now available.

EUnetHTA is pleased to announced that the document on “Patient Input in Relative Effectiveness Assessments” is now available. The document describes the process regarding direct patient input in REAs within JA3. The document is primarily intended for those who design and conduct EUnetHTA REAs, although it may be informative for a wider audience of patients, […]
HTAi 2019 Annual Meeting – Cologne!

This year’s HTAi Annual Meeting will take place from 15 to 19 June in Cologne, Germany. The meeting brings together HTA experts from across the world for five days of discussions, panels, presentations and more. This year’s theme “HTA beyond 2020: Ready for the New Decade” focusses on what the future development of HTA worldwide looks like as a number of prominent strategies and […]
PTJA04 – “Sotagliflozin indicated as an adjunct to insulin therapy to improve glycaemic control in adults with type 1 diabetes mellitus with a Body Mass Index (BMI) ≥ 27 kg/m2, who have failed to achieve adequate glycaemic control despite optimal insulin therapy” is now available
This is the assessment of the relative effectiveness of ‘Sotagliflozin indicated as an adjunct to insulin therapy to improve glycaemic control in adults with type 1 diabetes mellitus with a Body Mass Index (BMI) ≥ 27 kg/m2, who have failed to achieve adequate glycaemic control despite optimal insulin therapy.’ Below is the documentation provided by […]
Open Call for Patient Group Input – Collaborative Assessment on a Medical Device for Rectum Spacers for Prostate Cancer Radiotherapy

EUnetHTA recently started a new Collaborative Assessment on a Medical Device for rectum spacers for prostate cancer radiotherapy. To find out about participation, please read more here. Input submissions will be received through EOB, Friday 5th July.
Open Call for Patient Input – Joint Assessment on a medicinal product for neovascular (wet) age-related macular degeneration (AMD).
EUnetHTA recently started a new Joint Assessment on a medicinal product for neovascular (wet) age-related macular degeneration (AMD). To find out about participation, please read more here. Input submissions will be received through 13:00, July 15th.
OTCA18 Final Project Plan – Regional hyperthermia for high-risk soft tissue sarcoma treatment – now available

The Final OTCA18 Project Plan on ‘Regional hyperthermia for high-risk soft tissue sarcoma treatment’ is now available for access, together with comments from external experts, Haukeland Hospital, and manufacturers, and the relevant answers for each from the assessment team. Please access the Final Project Plan and related documentation via the following links: OTCA18 – Final […]
REQueST tool and its Vision paper Public Consultation – Now closed

The Haute Autorité de Santé (HAS), National Institute for Health and Care Excellence (NICE) & the Croatian Institute for Public Health (HZJZ) are pleased to share the following WP5 strand B draft outputs for an 8 week public consultation: REQueST (Registry Evaluation and Quality Standards Tool): a tool that is built upon the results of […]
OTCA11 Final Assessment – Now Available
We are pleased to announce that the collaborative rapid assessment, OTCA11, on “Custom-made or customisable 3D printed implants and cutting guides versus non-3D printed standard implants and cutting guides for improving outcome in patients undergoing knee, maxillofacial, or cranial surgery” is now available. This rapid assessment addresses the research question of whether 3D printed custom-made […]

